
Quick Summary
Table of Contents
Have you ever wondered why is the ocean salty while rivers and lakes usually contain fresh water? This simple question hides a fascinating story about Earth’s natural processes, from the weathering of rocks to the movement of water through the hydrological cycle.
Ocean salinity refers to the concentration of dissolved saltsbmainly sodium chloride in seawater. On average, the world’s oceans have a salinity of about 35 parts per thousand (ppt), or roughly 3.5% salt by weight. This means that for every liter of seawater, around 35 grams of salt are dissolved.
Understanding ocean salinity is more than just a matter of curiosity. It plays a vital role in marine ecosystems, influences global climate and ocean circulation, and directly affects human activities such as fishing, shipping, and even the availability of drinking water through desalination.

The saltiness of oceans is not a sudden phenomenon but the outcome of geological and chemical processes operating over billions of years. While rivers and lakes constantly cycle through land surfaces, the oceans act as the end-point reservoirs, collecting dissolved salts that rarely escape.
When ocean water evaporates under solar heat, only H₂O molecules rise as vapor. The dissolved salts, being non-volatile, are left behind. This is why rainwater is fresh even though it originates from salty seas. Over geological time, this unidirectional process has concentrated salts in oceans while keeping rivers and rainfall fresh.
The hydrological cycle plays a crucial role in maintaining salinity levels:
Scientists estimate that the average ocean salinity has stabilized at around 35 ppt (3.5%) because the rate of salt input (from rocks, rivers, and vents) is balanced by the rate of removal (through processes like mineral deposition, biological uptake, and seafloor sedimentation).
The question of why oceans are salty has fascinated scientists and philosophers for centuries. Before modern oceanography and geochemistry provided detailed explanations, several early theories attempted to answer this mystery.
The English astronomer Edmond Halley famous for Halley’s Comet was among the first to suggest a scientific explanation. In 1715, he proposed that the salts in the ocean come from rivers. According to his view:
Halley’s idea was groundbreaking because it shifted the explanation from religious or mythological beliefs to natural scientific processes.
In the late 19th century, the Irish scientist John Joly expanded on Halley’s hypothesis. He argued that if rivers continuously add salts to the ocean, then the age of the ocean could be calculated from its salt content. In 1899, Joly estimated that the oceans must be about 80 to 90 million years old.
With the rise of oceanography, geochemistry, and plate tectonics, scientists gained a more accurate understanding of salinity:
| Scientist / Period | Main Idea | Strengths | Limitations |
|---|---|---|---|
| Edmond Halley (1715) | Rivers carry salts to oceans; evaporation leaves salt behind | First natural scientific explanation | Didn’t explain long-term balance of salinity |
| John Joly (1899) | Ocean age can be estimated using salinity | Early attempt at using geochemistry to date Earth | Gave an incorrect age (~90 million years) |
| Modern View (20th C. →) | Salinity is in dynamic equilibrium (inputs = outputs) | Supported by oceanography, chemistry, and plate tectonics | More complex, requires advanced data |

The oceans are salty because of a continuous supply of minerals and ions from multiple natural sources. While rivers remain the primary contributors, other processes like volcanic activity and atmospheric inputs also play a role in maintaining ocean salinity.
Together, these sources explain why ocean salinity has remained stable at an average of 35 ppt (3.5%), despite constant water movement through the hydrological cycle.
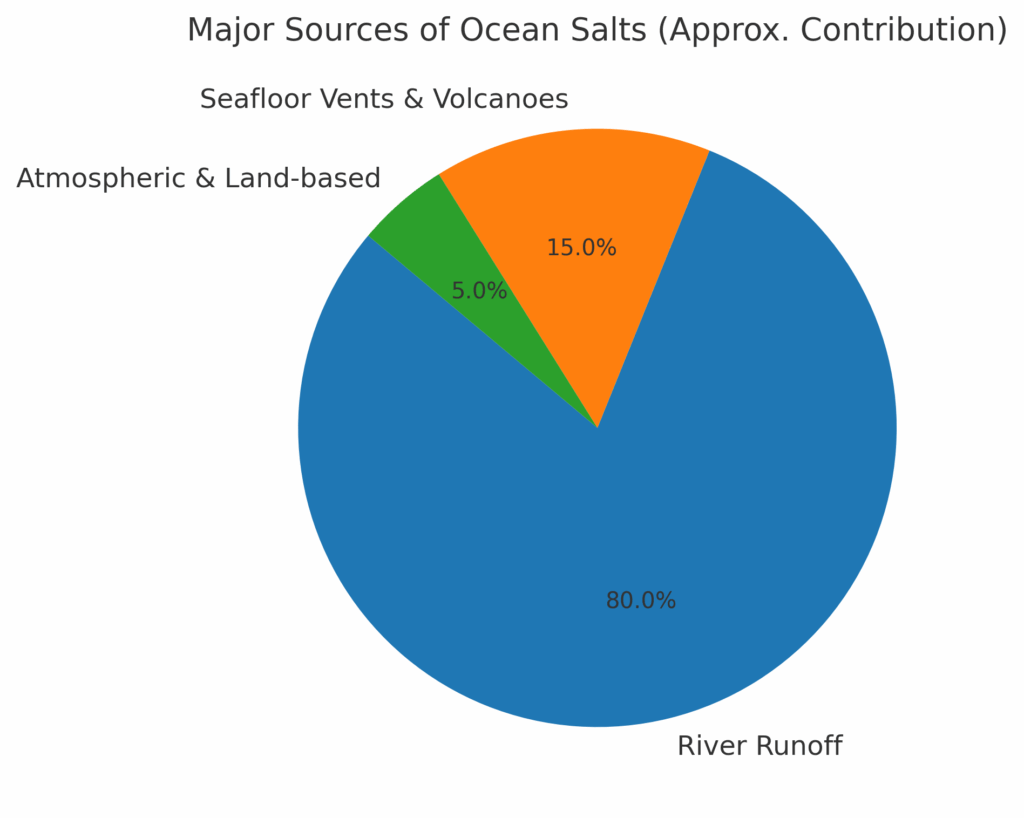
Here’s the pie chart showing the approximate contributions of different sources to ocean salts:
Ocean salinity is not uniform across the globe. It varies depending on climate, geography, and ocean circulation. These variations explain why some seas are saltier than others and why polar waters differ from tropical regions.
In short, climate, ice processes, basin geography, and circulation patterns together regulate how salty different parts of the ocean are.
A common question arises: if rivers and other sources are constantly adding salts to the oceans, why aren’t the oceans getting saltier over time? The answer lies in the dynamic balance between salt input and removal processes.
Together, these removal processes balance the continuous input of salts from rivers, volcanoes, and the atmosphere. This balance has kept the average ocean salinity stable at around 35 ppt (3.5%) for millions of years, making Earth’s oceans a remarkably steady environment for life.
Salinity not only shapes marine ecosystems but also reveals fascinating insights into Earth’s water systems. Here are some intriguing facts about ocean salt levels:

Ocean salinity is not just a measure of how salty seawater is it plays a critical role in shaping Earth’s climate, regulating marine ecosystems, and supporting human life. Understanding salinity helps scientists predict weather patterns, track climate change, and manage freshwater resources.
Salinity, together with temperature, drives the thermohaline circulation, also known as the “global conveyor belt” of ocean currents. Salty water is denser than fresh water; when combined with cooling in polar regions, it sinks and pushes deep-sea currents. These currents transport heat, nutrients, and gases around the planet, influencing:
Salinity levels directly shape marine habitats. Most open oceans maintain a range of 34–37 parts per thousand (ppt), but estuaries, polar seas, and enclosed basins can vary widely. These differences impact:
Ocean salinity also touches human civilization in practical ways:
The ocean is salty because of the continuous weathering of rocks, river inflow, and volcanic activity, processes that have been shaping Earth for billions of years. While rivers carry dissolved minerals into the sea, removal mechanisms like mineral deposition, subduction, and biological uptake ensure that the ocean’s saltiness remains relatively balanced over time.
This sea water salinity is not just a chemical property it is a driver of global climate, ocean circulation, marine ecosystems, and even human survival through resources like desalination and salt extraction. Without salinity, the ocean as we know it would not regulate Earth’s temperature or sustain the vast diversity of life it harbors.
The ocean’s saltiness is not just a mystery it’s a story of Earth’s history written in every drop.
Read Also:-
Ocean water is salty because rainwater erodes rocks, carrying dissolved salts and minerals through rivers into the sea. Over millions of years, these ions have accumulated in the ocean. While marine organisms use some, most remain, giving seawater its salinity.
In smaller lakes, ions circulate and exit more quickly, preventing significant salt buildup. Unlike oceans, where salts accumulate over millions of years, lakes usually lack enough time for such accumulation, which is why their waters remain fresh rather than salty.
No sea is entirely freshwater; all contain some salt. The Baltic Sea, the least salty, owes its low salinity to limited evaporation, cold climate, and abundant river inflow, yet it still remains a saltwater body rather than freshwater.
The Atlantic Ocean is the saltiest major ocean, with an average salinity of about 37 parts per thousand. High evaporation, warm temperatures, and lower river inflow compared to other oceans contribute to its greater salt concentration and overall salinity.
Ocean water is not safe to drink because its high salt content dehydrates the body instead of quenching thirst. Drinking seawater forces the kidneys to expel excess salt, leading to dehydration, organ strain, and potentially life-threatening health complications.
The average salinity of ocean water is about 35 parts per thousand (ppt), meaning 35 grams of dissolved salts per liter of seawater. Salinity varies across regions, with higher levels in warm, dry areas and lower levels near polar regions.
Salt in seawater forms mainly from the weathering of rocks on land. Rainwater dissolves minerals like sodium and chloride, carrying them through rivers into the ocean. Volcanic activity, seafloor vents, and atmospheric inputs also add salts, creating seawater’s characteristic salinity.

Authored by, Muskan Gupta
Content Curator
Muskan believes learning should feel like an adventure, not a chore. With years of experience in content creation and strategy, she specializes in educational topics, online earning opportunities, and general knowledge. She enjoys sharing her insights through blogs and articles that inform and inspire her readers. When she’s not writing, you’ll likely find her hopping between bookstores and bakeries, always in search of her next favorite read or treat.
Editor's Recommendations
Chegg India does not ask for money to offer any opportunity with the company. We request you to be vigilant before sharing your personal and financial information with any third party. Beware of fraudulent activities claiming affiliation with our company and promising monetary rewards or benefits. Chegg India shall not be responsible for any losses resulting from such activities.
Chegg India does not ask for money to offer any opportunity with the company. We request you to be vigilant before sharing your personal and financial information with any third party. Beware of fraudulent activities claiming affiliation with our company and promising monetary rewards or benefits. Chegg India shall not be responsible for any losses resulting from such activities.